News & Press
A Synthetic Counterpart for Dynamic Processes in Cells
Researchers recapitulate the mechanism by which membrane-less organelles form in cells
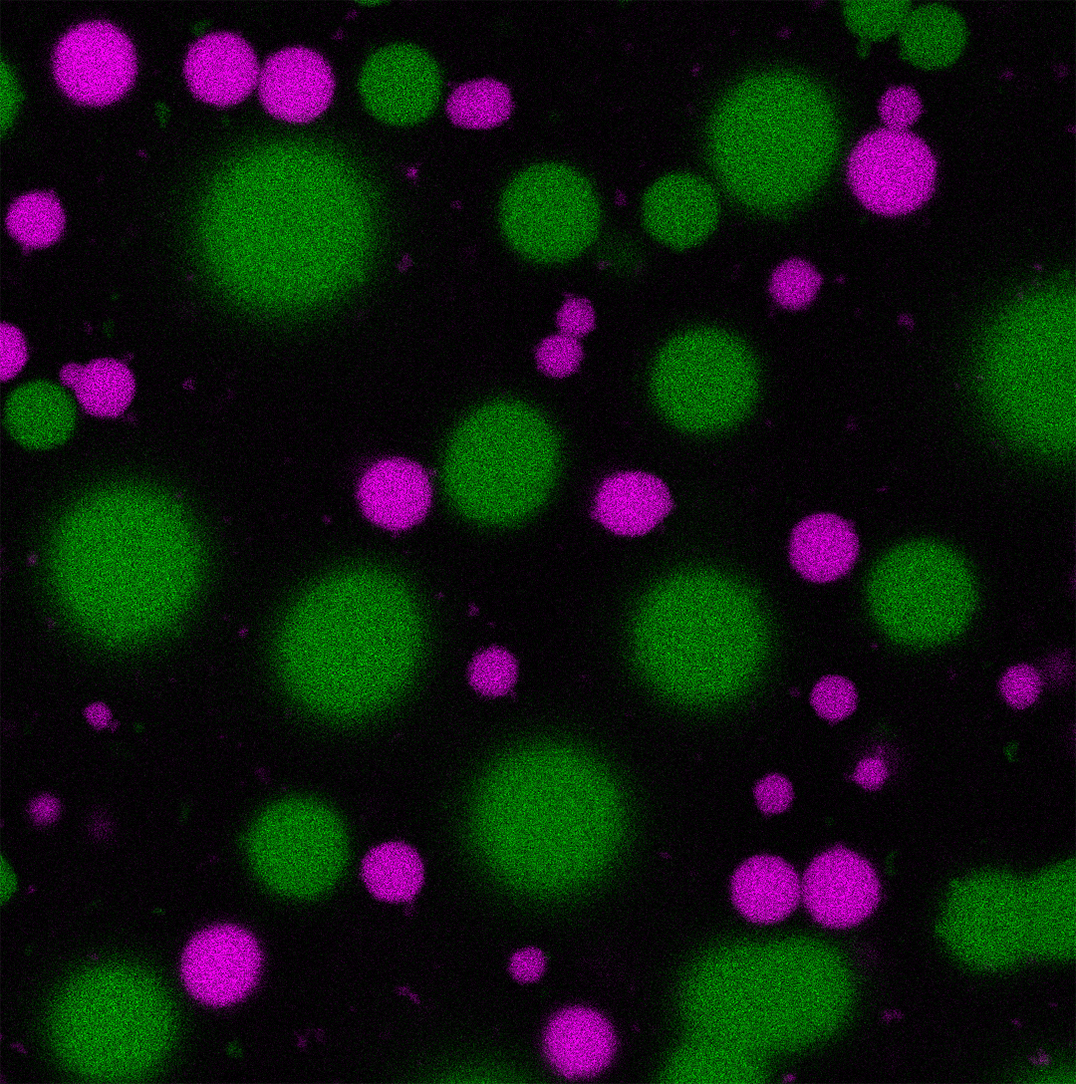
Fluorescence microscopy image of self-sorted DNA droplets, marked by two different fluorescent labels. Image: A. Walther/ livMatS
The large number of biochemical processes that occur in each living cell requires their finely tuned spatial and temporal organization. One ubiquitous process in cells is the concentration of biomolecules into membrane-less compartments through liquid-liquid phase separation (LLPS). These compartments can dynamically change in size, number and composition during the cell’s life cycle, and serve crucial functions such as the regulation of gene expression. Multivalent interactions between their molecular components are a key driving principle for the compartments’ dynamic changes.
Prof. Andreas Walther from the Cluster of Excellence “Living, Adaptive and Energy-autonomous Materials Systems (livMatS)” and Jie Deng from the Institute for Macromolecular Chemistry at the University of Freiburg created the first synthetic LLPS system with well-defined multivalency principles. This emulation of natural principles has a high potential for further use in fundamental research, chemical synthesis and the development of adaptive materials systems. Their results were published in the scientific journal Chem, a sister journal of Cell.
The researchers built on a previously described system, consisting of synthetic DNA strands and two types of enzymes with the ability to either cut DNA, or fuse DNA strands with the use of ATP as an energy source. The DNA forms droplets, in which the concurring fragmentation and elongation of DNA results in a non-equilibrium state with a set lifetime, defined by the availability of ATP molecules.
Through customization of the DNA strands, the researchers achieved self-sorting of different DNA-strands into separate droplets, allowing them to run several of these systems in parallel. By specifically attaching enzymes to the self-sorting DNA strands, spatially confined and separated reaction compartments can be created.
“In the future, these compartments may be used as reactors that can transiently catalyze chemical reactions”, says Jie Deng, who is first author of the study. “On the other hand, multivalency-driven LLPS may find applications in cell biology, such as regulation of receptor clustering during cellular signaling.”
Original Publication:
Jie Deng, Andreas Walther: Programmable ATP-Fueled DNA Coacervates by Transient Liquid-Liquid Phase Separation. In: Chem (2020), doi: doi.org/10.1016/j.chempr.2020.09.022
